Answer: Water must be present for this reaction to occur.
Step-by-step explanation:
Decomposition reactions require breaking of bonds which require energy and thus all of the decomposition reactions are endothermic reactions.
The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) whereas liquids are represented by (l) and gases are represented by (g) after their chemical formulas.
The balanced chemical reaction of
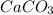 decomposition is:
decomposition is:
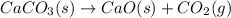
The decomposition of
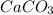 requires heat and leads to formation of CaO as solid and
requires heat and leads to formation of CaO as solid and
 as product.
as product.
Thus the the statement that is not true is Water must be present for this reaction to occur.