Answer:
The new temperature is 213.33 K
Step-by-step explanation:
Charles's law indicates that the volume of gas at constant pressure is directly proportional to its temperature. This law indicates that if the temperature increases the volume increases and if the temperature decreases the volume decreases.
So, Charles's law is a law that says that when the amount of gas and pressure are kept constant, the ratio between volume and temperature will always have the same value:
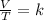
When you want to study two different states, an initial and a final one of a gas, the expression can be applied:
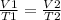
In this case, you know:
- V1= 15 L
- T1= 640 K
- V2= 5 L
- T2=?
Replacing:
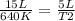
Solving:
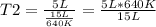
T2= 213.33 K
The new temperature is 213.33 K