Answer:
C. 7.4 kJ
Step-by-step explanation:
Let assume that Mercury is at room temperature (25 °C). The energy required to boil the sample of mercury is the sum of sensible and latent heats. Mercury has a fussion and boiling points of -38.83 °C and 356.7 °C, respectively, and a specific heat of
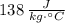 . Then:
. Then:
![Q = (25\,g)\cdot \left[\left(0.138\,(J)/(g\cdot ^(\circ)C) \right)\cdot (356.7^(\circ)C - 25^(\circ)C) + 296\,(J)/(g) \right]](https://img.qammunity.org/2021/formulas/chemistry/high-school/1g18vy55cwlzjm7cqrottgz0auxpls10yd.png)
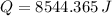
The option that offers the best approximation to the result is C.