Answer:

Step-by-step explanation:
Hello,
In this case, for water, whose molar mass is 18 g/mol, we can find two moles of hydrogen in one mole of water, therefore, for us compute the atoms, we should also use the Avogadro's number as shown below:
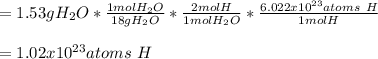
Regards.