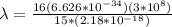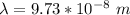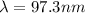Answer:
The wavelength is
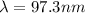
Step-by-step explanation:
Generally the series whose emission line show on the visible spectrum is the
Balmar series so this two emission line seen on the visible spectrum could either be due to the move of electron from
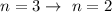
OR

This implies that the first excitement is from
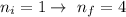
So the energy change due to the excitement is mathematically represented as
![\Delta E = R_H [(1)/(n_i^2) -(1)/(n_f^2) ]](https://img.qammunity.org/2021/formulas/chemistry/college/e76stvvperhh1eddrwil9wovxbpzxbycya.png)
substituting values
![\Delta E= R_H [(1)/(1^2) -(1)/(4^2) ]](https://img.qammunity.org/2021/formulas/chemistry/college/hpnxfeekiekv9rbxgloawxrin8xwoxhaq4.png)
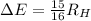
This energy change can also be represented as
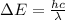
So
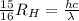
=>
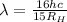
Where
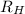 is the Rydberg constant with a value of
is the Rydberg constant with a value of
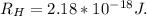
h is the Planck's constant with values
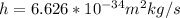
c is the speed of light with value
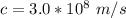
So