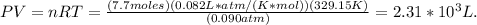Answer:
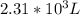 of gas are in the container.
of gas are in the container.
Step-by-step explanation:
1. First, convert temperature to kelvins. Otherwise, you will get the wrong answer. T = 56 + 273.15 = 329.15 K.
2.Use the ideal gas law.
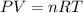
3.Re-arrange for volume and use the gas contstant R =
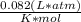
4.Insert values.