Answer:
1.42 M
Step-by-step explanation:
In this case have a dilution problem, therefore we need to use the dilution equation:
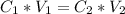
What values we have?
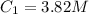
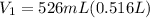
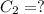
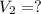
Now, we can calculate
 if we add the volumes, so:
if we add the volumes, so:
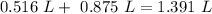
So,
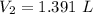
We can plug the values in the equation:
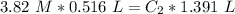
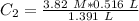
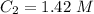
I hope it helps!