Answer:
1.77 atm
Step-by-step explanation:
We have to check the values that gives the problem:
V= 70 L
mass =354.5 g
Molas weight= 70.9 g/mol
T=30 ºC
P= ?
We can find the moles of chlorine if we use the molar weight:
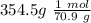
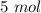
Now, we have the moles, volume, temperature therefore we can use the ideal gas equation:
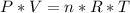
We know the R value:
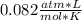
We have “K” units for the temperature, so we need to do the conversion:
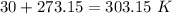
With all the data we can plug the values into the equation:
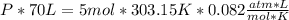
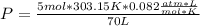
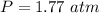
I hope it helps!