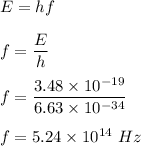Answer:
Frequency,

Step-by-step explanation:
It is given that, a yellow laser provides 210 kJ/mol of energy to excite electrons in a sample. We need to find the frequency of this light.
Energy, E = 210 kJ/mol
i.e.
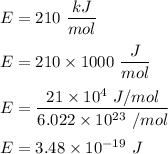
The energy used to excite electrons is given by :