Answer:
Approximately
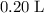 .
.
Step-by-step explanation:
Convert the temperature of this gas to absolute temperature:
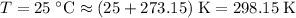 .
.
Let
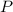 and
and
 represent the pressure and volume of this gas, respectively. Let
represent the pressure and volume of this gas, respectively. Let
 represent the number of gas particles in this gas. Let
represent the number of gas particles in this gas. Let
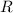 represent the ideal gas constant. By the ideal gas law:
represent the ideal gas constant. By the ideal gas law:
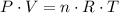 .
.
For this question:
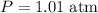 (given,)
(given,)
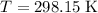 (from unit conversion,) and
(from unit conversion,) and
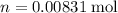 .
.
Look up the ideal gas constant
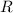 that takes
that takes
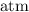 as the unit for pressure:
as the unit for pressure:
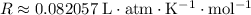 .
.
This question is asking for
 , the volume of this gas. Rearrange the ideal gas equation and solve for
, the volume of this gas. Rearrange the ideal gas equation and solve for
 :
:
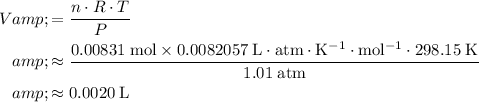 .
.