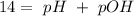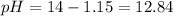Answer:
pH =1 2.84
Step-by-step explanation:
First we have to start with the reaction between HCl and KOH:
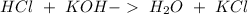
Now for example, we can use a volume of 10 mL of HCl. So, we can calculate the moles using the molarity equation:
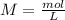
We know that
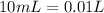 and we have the concentration of the HCl
and we have the concentration of the HCl
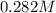 , when we plug the values into the equation we got:
, when we plug the values into the equation we got:
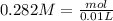
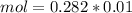
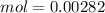
We can do the same for the KOH values (
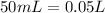 and
and
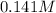 ).
).
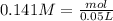
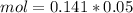
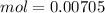
So, we have so far 0.00282 mol of HCl and 0.00705 mol of KOH. If we check the reaction we have a molar ratio 1:1, therefore if we have 0.00282 mol of HCl we will need 0.00282 mol of KOH, so we will have an excess of KOH. This excess can be calculated if we substract the amount of moles:
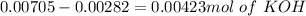
Now, if we want to calculate the pH value we will need a concentration, in this case KOH is in excess, so we have to calculate the concentration of KOH. For this, we already have the moles of KOH that remains left, now we need the total volume:
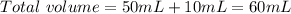
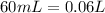
Now we can calculate the concentration:
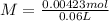
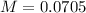
Now, we can calculate the pOH (to calculate the pH), so:
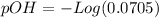
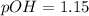
Now we can calculate the pH value: