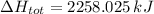Answer:
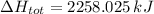
Step-by-step explanation:
The amount of heat released from water is equal to the sum of latent and sensible heats. Let suppose that water is initially at a temperature of
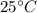 . Then:
. Then:
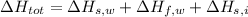
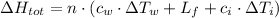
Finally, the amount of heat released from water is now computed by replacing variables:
![\Delta H_(tot) = (1\,mol)\cdot \left[\left(75.3\,(kJ)/(mol\cdot K) \right)\cdot (25^(\circ)C-0^(\circ)C)+ 6.025\,(kJ)/(mol) + \left(37.7\,(kJ)/(mol\cdot K) \right)\cdot (0 + 10^(\circ)C)\right]](https://img.qammunity.org/2021/formulas/physics/college/mcsc8332t3pmb7i5dr0sijbvjsj10aes3n.png)