Answer:
(D) - 16.6 kcal
Step-by-step explanation:
Hello,
In this case, the Gibbs free energy for the given reaction is computed in terms of the Gibbs free energy of formation of each species involved in the chemical reaction:
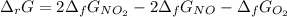
Thus, it is found for nitrogen monoxide, oxygen and nitrogen dioxide the following Gibbs free energies of formation: 87.6, 0 and 51.3 kJ/mol respectively, therefore we compute:
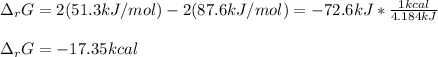
The closest result is (D) - 16.6 kcal, as such difference is noticed when different sources for thermochemical data are used, in this case, the NIST data were used.
Best regards.