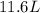Answer:
The new volume is
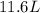
Step-by-step explanation:
This problem is on the application of Boyle's law which states that" the volume of a given mass of gas at constant temperature is inversely proportional to the pressure"
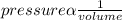
Given data
initial pressure p1= 100atm
initial volume v1= 3.7L
final pressure p2= 32 atm
final volume v2= ?
Apply Boyle's law we have
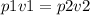
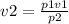
Substituting our data we have
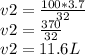
The new volume is