Answer:
Option f: an addition of HCl will exceed the buffer capacity. The option d is also correct since it is a consequence of the option f.
Step-by-step explanation:
The pH of the buffer solution before the addition of HCl is:
![pH = pKa + log(([KF])/([HF]))](https://img.qammunity.org/2021/formulas/chemistry/college/pbk8qmebgna803clr5byzhljul1f4ya9j9.png)
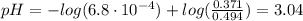
The hydrochloric acid added will react with the potassium fluoride as follows:
H₃O⁺(aq) + F⁻(aq) ⇄ HF(aq) + H₂O(l)
The number of moles (η) of potassium fluoride (KF) and the HF before the addition of HCl is:
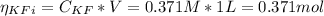
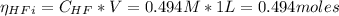
The number of moles of the HCl added is 0.408 moles. Since the number of moles of HCl is bigger thant the number of moles of KF, the moles of HCl that remains after the reaction is:
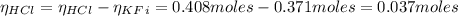
Hence, the KF is totally consumed after the reaction with HCl and thus, exceding the buffer capacity.
We can calculate the pH after the addition of HCl:
HF(aq) + H₂O(l) ⇄ F⁻(aq) + H₃O⁺(aq) (1)
The number of moles of HF after the reaction of KF with HCl is:
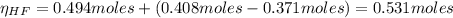
And the concentration of HF after the reaction of KF with HCl is is:
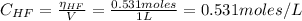
Now, from the equilibrium of equation (1) we have:
![Ka = ([H_(3)O^(+)][F^(-)])/([HF])](https://img.qammunity.org/2021/formulas/chemistry/college/3c6zvs1en7spf70hrh8l2x3oya03d7d3rq.png)
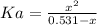 (2)
(2)
By solving equation (2) for x we have:
x = 0.0187
Finally, the pH after the addition of HCl is:
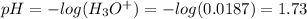
Therefore, the addition of HCl will exceed the buffer capacity and thus, lower the pH by several units. The correct option is f: an addition of HCl will exceed the buffer capacity. The option d is also correct since it is a consequence of the option f.
I hope it helps you!