Answer:
8.0 mol/L
Step-by-step explanation:
To do this problem you need to use C₁V₁=C₂V₂. C=concentration and V=volume. (Note: you dont want to use M₁V₁=M₂V₂ because this is measuring mass not concentration).
So, V₁= 1.0L
C₂=2 Mol/L
V₂= 4 L
C₁ is unknown.
you would plug this equation in like this:
(C₁)(1.0 L)=(2.0 Mol/L)(4.0L).
Do algebra to get C₁ alone.
C₁=
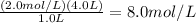
The liters cancel out and you are left with mol/L or M
I hope this helps! good luck :)