Answer: 4.74 moles of gas are in the container.
Step-by-step explanation:
1. Use the ideal gas law. PV = nRT. and re-arrange from moles by divided RT on both sides.
2.You should get the following equation:
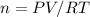
3.Insert values:
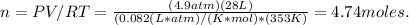
4.There are 4.74 moles in the container.
Good luck with chemistry!