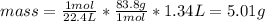Answer:
5.01 g
Step-by-step explanation:
At STP means at standard temperature and pressure, this is when the pressure is at 1 atm and temperature is at 273 K. At this point, the volume is 22.4 L for 1 mole of any ideal gas at a temperature equal to 273.15 K and a pressure equal to 1.00 atm
The molar mass of krypton as gotten from the periodic table is 83.8 g/mol. At STP, an ideal gas occupies 22.4 L/mol. Therefore, the grams that 1.3 L of krypton gas would occupy is: