Answer:
860.6 years.
Step-by-step explanation:
The parameters given are;
Initial detector activity = 370000 alpha decays per second
Final detector activity = 93000 alpha decays per second
Formula for time to change in activity is given by the following relation;
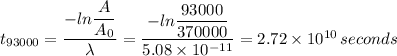
t₉₃₀₀₀ = 2.72 × 10¹⁰ seconds = 860.6 years.