Answer:
a) CO is the limiting reactant.
b)
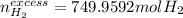
c)
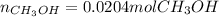
Step-by-step explanation:
Hello,
In this case, the balanced chemical reaction:
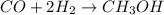
So we proceed as follows:
a) We first compute the moles of CO by using its density (0.00114 g/mL) and molar mass (28 g/mol):
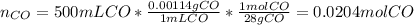
Next, with the given 750 moles of hydrogen, we can compute the moles of carbon monoxide that are consumed by such amount of hydrogen by using their 1:2 molar ratio:
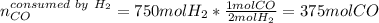
Thus, we see a clear excess of hydrogen, for that reason the carbon monoxide is the limiting reactant.
b) In this case, we first compute the moles of CO that are not consumed:
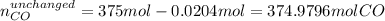
Next, we use the 1:2 molar ratio again to compute the unchanged moles of hydrogen which is the excess reactant:
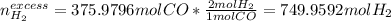
c) Finally, we use the reacting moles of carbon monoxide to compute the formed moles of methanol by using the 1:1 molar ratio:
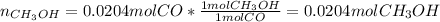
Best regards.