Answer:
0.92787 liters of bromine are needed to produce 12 moles of aluminum bromide.
Step-by-step explanation:
You have the following balanced equation:
2 Al (s) + 3 Br₂ (l) ⇒ 2 AlBr₃ (s)
First of all, the following rule of three should be applied to know the amount of moles of bromine needed: if 2 moles of aluminum bromide are produced by stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction) for 3 mole of bromine, 12 moles of aluminum bromide with how many moles of bromine are produced?
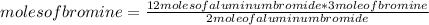
moles of bromine= 18
Being the molar mass of the bromine Br₂ 159.8 g/mol then the mass of 18 moles of Br₂ is:
18 moles* 159.8 g/mol= 2,876.4 grams
Density is a property that indicates the amount of mass per unit volume. Then the following rule of three applies: if by the definition of density 3.1 grams of bromine are present in 1 mL, 2,876.4 grams of bromine are present in how much volume is it?
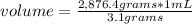
volume= 927.87 mL
Being 1,000 mL= 1 L, then 927.87 mL= 0.92787 L
0.92787 liters of bromine are needed to produce 12 moles of aluminum bromide.