Answer:
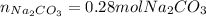
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
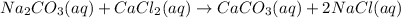
Hence, given the solution of calcium chloride, we can compute its reacting moles:
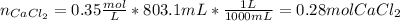
Thus, by knowing there is a 1:1 molar ratio between sodium carbonate and calcium chloride, we can easily compute the moles of sodium carbonate needed for a complete precipitation as shown below:
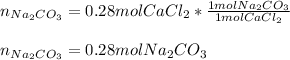
Best regards.