Answer:
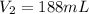
Step-by-step explanation:
Hello,
In this case, for dilution processes, the initial moles must be the same to the final moles, therefore, only the volumes and molarities change from the beginning to the end of the dilution:

In this case, we are asked to compute the new or final volume, so we simply solve for it as shown below:
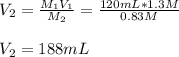
Best regards.