Answer:
1.11 L
Step-by-step explanation:
Given data
- Molarity of the oxalic acid solution (M): 0.871 M
- Mass of oxalic acid (m): 86.9 g
Step 1: Calculate the moles corresponding to 86.9 g of oxalic acid
The molar mass of oxalic acid is 90.03 g/mol.
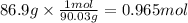
Step 2: Calculate the volume of a 0.871 M solution that contains 0.965 moles of oxalic acid
The molarity is equal to the moles of solute (n) divided by the volume of the solution (V).
