Answer:
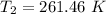
Step-by-step explanation:
It is given that,
Original temperature,
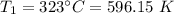
Original volume,
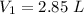
We need to find the temperature if the volume of the balloon to be shrink to 1.25 L.
According to Charles law, at constant pressure,
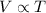
It would means,
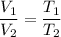
T₂ = ?
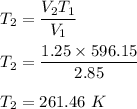
So, the new temperature is 261.46 K.