Answer:
The concentration of hydronium ions and the pH value is related by the equation: pH=-Log[H+]
Step-by-step explanation:
We have two different concepts pH and concentration of hydronium ions. Lets start with the hydronium ion.
An hydronium ion is a species that is produced by an acid:
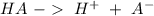
Aditionally, we can have the production of hydroxide ions. The subtances that have the capacity to produce this ions are called "bases":
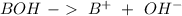
Now we can continue "pH"
The pH is a scale that indicates if the substance is and acid (higher concentration of
 ), neutral (equal amounts of
), neutral (equal amounts of
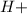 and
and
 ) or basic (higher amount of
) or basic (higher amount of
 ).
).
Finally, the "pH" is calculated with the concentration of the hydronium ions (
 ), the letter "p" is "-Log", therefore:
), the letter "p" is "-Log", therefore:
![pH=-Log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/ou2r3i8avxjbtojbzowuc2df0qtmcfaztp.png)
I hope it helps!