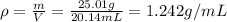Answer:
1.242 g/mL
Step-by-step explanation:
Step 1: Given data
Mass of the empty container (m₁): 80.21 g
Mass of the filled container (m₂): 105.22 g
Volume of the unknown liquid (V): 20.14 mL
Step 2: Calculate the mass of the liquid
The mass of the liquid is equal to the difference between the mass of the filled container and the mass of the empty container.
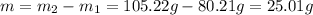
Step 3: Calculate the density of the unknown liquid
The density of the liquid is equal to its mass divided by its volume.