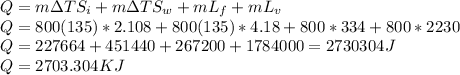Answer:
2730.304 KJ
Step-by-step explanation:
How much heat is required to convert 0.8 kg of ice at -35°C into steam at 100 C?
Given that:
mass of ice (m) = 0.8 kg = 800 g
Initial temperature (
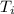 ) = -35°C = 238 K
) = -35°C = 238 K
final temperature (
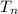 )= 100°C = 373 K
)= 100°C = 373 K
Specific heat of ice (
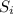 ) = 2.108 J/g.K
) = 2.108 J/g.K
Specific heat of water (
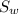 ) = 4.18 J/g.K
) = 4.18 J/g.K
Latent heat of fusion (
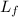 ) = 334 J/g.
) = 334 J/g.
Latent heat of vaporization (
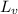 ) = 2230 J/g.
) = 2230 J/g.
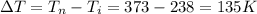
Total heat (Q) required to increase the temperature of ice from the initial temperature of 238K to final temperature of 373 K is given by the equation: