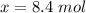Answer:
The correct answer to the following question will be Option B "8.4 mol".
Step-by-step explanation:
The given values are:
Molarity of a solution = 0.021 M
Litres of solution = 400 L
Let the moles be "x".
As we know,
⇒
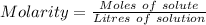
On putting the estimated values, we get
⇒
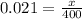
On applying cross-multiplication, we get
⇒
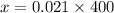
⇒