Answer:
The equation for the Kinetic Energy,
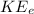 , of the photo-emitted electrons is given as follows;
, of the photo-emitted electrons is given as follows;
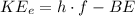
Please find attached the required plot of the kinetic energy versus the frequency
Step-by-step explanation:
The equation for the Kinetic Energy,
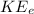 , of the photo-emitted electrons is given as follows;
, of the photo-emitted electrons is given as follows;
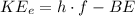
Where:
h = Planck's constant = 6.63×10⁻³⁴ J·s
f = Frequency
BE = Binding Energy = 2.71 eV for Calcium metal
f₀ = Threshold frequency for the material
The ionization energy is the energy required to free an electron from an isolated atom