Answer:
The molarity is 0.4
Step-by-step explanation:
The Molarity (M) or Molar Concentration is a concentration unit that indicates the number of moles of the solute per liter of solution. In other words, molarity is defined as the number of moles of solute that are dissolved in a given volume.
The Molarity of a solution is determined by the expression:
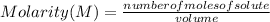
Molarity is expressed in units (
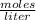 ).
).
In this case:
- number of moles of solute (sodium hydroxide)= 0.2 moles
- volume= 0.5 L
Replacing:
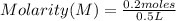
Molarity= 0.4
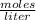
The molarity is 0.4