Answer:
2.25 atm
Step-by-step explanation:
Given data
- Total pressure: 10.46 atm
Step 1: Convert the pressure of nitrogen to atm
We will use the relationship 1 atm = 101.325 kPa.
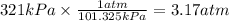
Step 2: Convert the pressure of oxygen to atm
We will use the relationship 1 atm = 14.6959 psi.
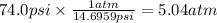
Step 3: Calculate the partial pressure of hydrogen
The total pressure is the sum of the partial pressures.
P = pN₂ + pO₂ + pH₂
pH₂ = P - pN₂ - pO₂
pH₂ = 10.46 atm - 3.17 atm - 5.04 atm
pH₂ = 2.25 atm