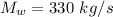Answer:
The minimum mass flow rate will be "330 kg/s".
Step-by-step explanation:
Given:
For steam,
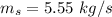
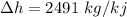
For water,
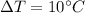
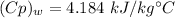
They add energy efficiency as condenser becomes adiabatic, with total mass flow rate of minimal vapor,
⇒
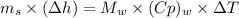
On putting the estimated values, we get
⇒
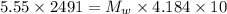
⇒
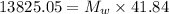
⇒