Answer:
About 8.94.
Step-by-step explanation:
Because we are given a 500. mL volumetric flask, the solution will have a volume of 500. mL.
Find the number of moles of zinc fluoride needed. Recall that molarity is simply moles per liter of solution:
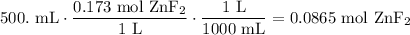
Convert this to grams. The molecular weight of zinc fluoride is 103.38 g/mol:
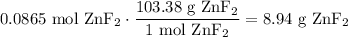
In conclusion, about 8.94 grams of solid zinc fluoride should be added.