Answer:
The new pressure is 21.19 atm.
Step-by-step explanation:
Original volume of the gas was 21 L at 11.1 atm
Neon in a piston is compressed to a volume of 11 L.
It is required to find the new pressure in atm. Boyle's law gives the relationship between volume and pressure. Its mathematical form is given by :
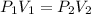
We have,
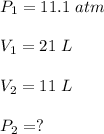
Using above equation,
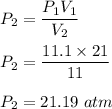
So, the new pressure is 21.19 atm.