Answer:
The new temperature is 200 K.
Step-by-step explanation:
Charles law gives the relationship between temperature and volume of a gas. It states that volume is directly proportional to temperature such that,
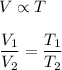
We have,
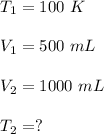
Plugging all the values,
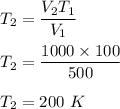
So, the new temperature is 200 K.