Answer:
The new volume of the gas is 29.57 Liters
Step-by-step explanation:
Given;
initial pressure of gas, P₁ = 12 atm
initial volume of gas, V₁ = 23 Liters
initial temperature of gas, T₁ = 200 K
final pressure of gas, P₂ = 14 atm
final temperature of gas, T₂ = 300 K
final volume of gas, V₂ = ?
To determine the final volume of the gas, we apply general gas law;
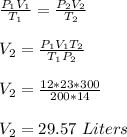
Therefore, the new volume of the gas is 29.57 Liters