Answer:
See the answer below
Step-by-step explanation:
When a solution of iodine is mixed with a solution of tetrachloromethane, the mixture turns purple due to the formation of
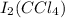 .
.
Iodine solution dissolves in carbon tetrachloride solution (another name for tetrachloromethane) due to the fact that both solutions are similarly non polar in nature. The equation of the reaction is as shown below:
