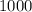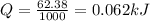Answer:
The Quantity of heat require is
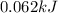
Explanation:
This problem bothers on the topic of heat capacity.
Given data
mass of water m=
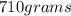
converting from grams to kg we have =
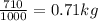
initial temperature T1= 4°C
final temperature T2= 25°C
heat capacity of water =
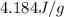
The expression for the quantity of heat required is given has
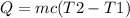
substituting our given data we have
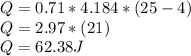
In kilograms we have to divide by