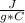Answer:
The specific heat capacity is 0.67
Step-by-step explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
The amount of heat that a body receives or transmits is determined by the following expression:
Q = c * m * ΔT
Where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
This expression indicates that between heat and temperature there is a direct proportional relationship.
In this case:
- Q= 100 J
- c= ?
- m= 5 g
- ΔT= Tfinal - Tinitial= 40 °C - 10 °C= 30 °C
Replacing:
100 J= c*5 g* 30°C
Solving:
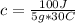
c= 0.67
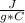
The specific heat capacity is 0.67