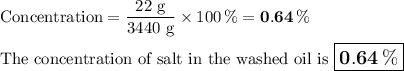Answer:

Step-by-step explanation:
Assume you are using 1 L of water.
Then you are washing 4 L of salty oil.
1. Calculate the mass of the salty oil
Assume the oil has a density of 0.86 g/mL.
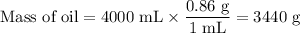
2. Calculate the mass of salt in the salty oil
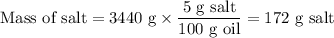
3. Calculate the mass of salt in the spent water
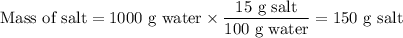
4. Mass of salt remaining in washed oil
Mass = 172 g - 150 g = 22 g
5. Concentration of salt in washed oil