Answer:
2,601 years
Explanation:
Given the decay model for carbon-14
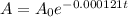
We want to determine the age of a skeleton that has lost 27% of its C-14.
Initial Value of C-14=100%=1
Present Amount, A(t)=100%-27%=73%=0.73
Substituting these into the model, we have:

The skeleton is approximately 2,601 years old.