Answer:
 atm L
atm L
Step-by-step explanation:
Complete question
A mixture of krypton and oxygen gas is expanded from a volume of 23.0L to a volume of 81.0L, while the pressure is held constant at 21.0atm. Calculate the work done on the gas mixture. Be sure your answer has the correct sign (positive or negative) and the correct number of significant digits.
Solution
As we know that -
Work done is equal to the product of pressure applied and change in the difference of volume of two liquids.
This can be represented mathematically as follows -
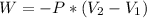
Where W represents the work done
P represents the change in pressure
 is the final volume and
is the final volume and
 is the initial volume
is the initial volume
Substituting the given values in above equation, we get -
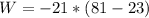
 atm L
atm L