Answer:
The cup with 0.5L
Step-by-step explanation:
To know what amount of water you take into account the specific heat of the water. The specific heat of water is:
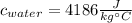
Thus, 4186 J of energy are needed to icrease the temperature of 1 kg water in 1°C. Then, more grams of water will need more energy.
You have that one cup has 0.5 L and the other one has 750mL = 0.75L
The second cup of water will need more heat because the amount of water contained in the second cup is greater than in the first cup with 0.5L