Answer:
Average atomic mass of element X is 56.19 amu
Step-by-step explanation:
Given:
Four isotopes of X:
5.845% of X has a mass of 53.93961 amu.
91.75% of X has a mass of 55.93494 amu.
2.123% of X has a mass of 56.93539 amu.
0.2820% of X has a mass of 57.93328 amu.
To find: average atomic mass of element X
Solution:
Isotopes are variants of a particular chemical element such that they have the same number of protons but different number of neutrons.
Average atomic mass of element X =
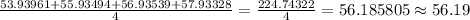
So, the average atomic mass of element X is 56.19 amu.