Answer:
1a. Both sides of the decay reaction have the same charge.
b. The number of nucleons on both sides are the same.
2. The binding energy of one mole of the atom is 17.172 ×
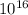 J.
J.
Step-by-step explanation:
1a. Considering the two sides of the decay reaction and with respect to the law of conservation of charge, it can be observed that both sides have the same charge. Charge can not be created or destroyed in the process.
b. The number of nucleons on both sides are equal. No nucleon is created or destroyed in the process.
2. Binding energy is the minimum energy required to separate an atom into its nucleons. From Einstein's energy equation;
E = Δm
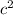
Where E is the binding energy of the atom, Δm is the mass defect and c is the speed of light.
Given that: Δm = 1.908 g/mol and c = 3 ×
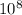 . So that:
. So that:
E = 1.908 ×
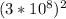
= 1.908 × 9 ×
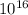
= 17.172 ×
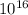 J
J
The binding energy of one mole of the atom is 17.172 ×
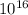 J.
J.