Answer:
Zr
Step-by-step explanation:
The balance equation of the reaction is:
Zr + 2 H₂O ⇒ ZrO₂ + 2 H₂
Being:
- Zr: 91 g/mole
- H: 1 g/mole
- O: 16 g/mole
the molar mass of the reactants participating in the reaction are:
- Zr: 91 g/mole
- H₂O: 2*1 g/mole + 16 g/mole= 18 g/mole
If by stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction) they react in moles:
then by stoichiometry the following quantity of mass react:
- Zr: 1 mole*91 g/mole= 91 g
- H₂O: 2 moles*18 g/mole= 36 g
Now the following rule of three applies: if 91 g of Zr react with 36 g of H₂O, 4500 g of Zr with how much mass of H₂O would it react?
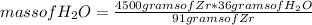
mass of H₂O= 1,780 grams
But 1780 grams of H₂0 are not available, 1.5 * 10⁴(15,000) grams are available. Since it has more mass than it needs to react with 4500 grams of Zr, Zr zirconia will be the limiting reagent.