Answer:
1.
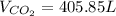
2.
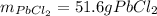
Step-by-step explanation:
Hello,
1) In this case, the reaction is:

Thus, since 1.595 kg of calcium carbonate, whose molar mass is 100 g/mol, is decomposed, the following moles of carbon dioxide are produced:
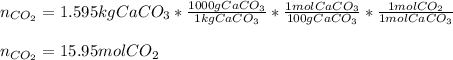
Then, by using the ideal gas equation we can compute the volume of carbon dioxide:
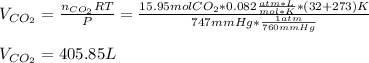
2) In this case, the reaction is:
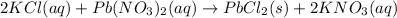
Thus, lead (II) chloride is the precipitate. In such a way, we first identify the limiting reactant by firstly computing the moles of potassium chloride and lead (II) nitrate with the volumes and the molarities:
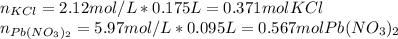
Next, we compute the moles of potassium chloride consumed by 0.567 moles of lead (II) nitrate by using their 2:1 molar ratio:
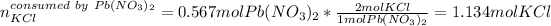
Therefore, as just 0.371 moles of potassium chloride are available we say it is the limiting reactant, for that reason, the grams of lead (II) chloride, whose molar mass is 278.1 g/mol, precipitate finally result:
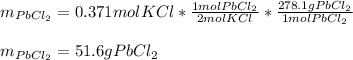
Best regards.