Answer:
A i. E = 9.62 × 10⁻⁷ J/s
ii. The absorbed dose is 4.81 × 10⁻⁶ Gy
iii. The equivalent dose is 3.37 × 10⁻⁴ rem/s
iv. t = 593471.81 seconds
B. i. 4.025 × 10¹⁵/s
ii. 0.512 mW
C. 7218092.2 seconds
D. i. 6.3 × 10⁻¹ J
ii. 1.4 × 10⁻² W
iii. 1.57 × 10³ Curie
E. 0.129 Ω
Step-by-step explanation:
The given parameters are;
Mass of tumor = 0.20 kg
Activity of Cobalt-60 = 2.60 × 10⁻⁴ Ci
Photon energy = 1.25 MeV
(i) The energy, E, delivered to the tumor is given by the relation;
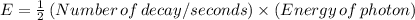
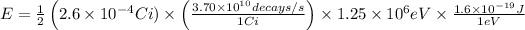
E = 9.62 × 10⁻⁷ J/s
(ii) The equation for absorbed dose is given as follows;
Absorbed dose, D, in Grays Gy = (Energy Absorbed Joules J)/Mass kg
Therefore, absorbed dose = (9.62 × 10⁻⁷ J/s)/( kg) = 4.81 × 10⁻⁶ Gy
1 Gray = 100 rad
4.81 × 10⁻⁷ Gy = 100 × 4.81 × 10⁻⁶ = 4.81 × 10⁻⁴ rad/s
(iii) Equivalent dose, H, is given by the relation;
H = D × Radiation factor,
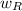
∴ H = 0.7 × 4.81 × 10⁻⁴ rad/s = 3.37 × 10⁻⁴ Sv = 3.37 × 10⁻⁴ rem/s
(iv) The exposure time required for an equivalent dose of 200 rem is given as follows;
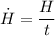
Therefore;

∴ t = 6.9 days
B. The number of electrons ejected is given by the relation;
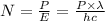

(ii) The power carried by the electron
The energy carried away by the electrons is given by the relation;
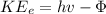
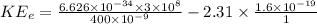
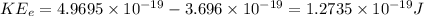
Power, P
 , carried away by the electron = 4.025 × 10¹⁵ × 1.2735 × 10⁻¹⁹ = 0.512 mW
, carried away by the electron = 4.025 × 10¹⁵ × 1.2735 × 10⁻¹⁹ = 0.512 mW
C. The given parameters are;
d = 1.19 mm, ∴ r = 1.19/2 = 0.595 × 10⁻³ m
l = 50 mm = 5 × 10⁻³ m
V = 500 ml = 5 × 10⁻⁴ m³
η = 0.0027 Pa
p = 1,900 Pa.
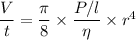
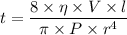
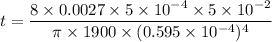
t = 7218092.2 seconds
D) i. Energy absorbed is given by the relation;
E = m×D
Where:
D = 35 Gray = 35 J/kg
m = 18 g = 18 × 10⁻³ kg
∴ E = 35 × 18 × 10⁻³ = 6.3 × 10⁻¹ J
ii. Total time for treatment = 15 × 5 = 75 minutes
Energy absorbed = 6.3 × 10⁻¹ × 100 = 63 J
Power = Energy(in Joules)/Time (in seconds)
∴ Power = 63/(75×60) = 1.4 × 10⁻² W
iii. Whereby the power is provided by 0.5% of the photons emitted by the source, we have;
1 MeV = 1.60218 × 10⁻¹³ J
0.03 MeV = 0.03 × 1.60218 × 10⁻¹³ J = 4.80654 × 10⁻¹⁵ J/photon
Therefore, the number of disintegration per second = 0.28 J/s ÷ 4.80654 × 10⁻¹⁵ J/photon = 5.83 × 10¹³ disintegrations per second
1 Curie = 3.7 × 10¹⁰ disintegrations per second
Hence, 5.83 × 10¹³ disintegrations per second = (5.83 × 10¹³)/(3.7 × 10¹⁰) Curie
= 1.57 × 10³ Curie
E. The parameters given are;
Density of water = 1000 kg/m³
Volume of water = 250 ml = 0.00025 m³
Initial temperature, T₁, = 25°C
Final temperature, T₂, = 100°C
Change in temperature, ΔT = 100 - 25 = 75°
Specific heat capacity of the water = 4200 J/kg/°C
Mass of water = Density × Volume = 1000 × 0.00025 = 0.25 kg
∴ Heat supplied = 4200 × 0.25 × 75 = 78,750 J
Time to heat the water = 45.0 sec
Therefore, power = Energy/time = 78750/45 = 1750 W
The formula for electrical power = I²R =VI = V²/R
Therefore, where V = 15.0 V, we have;
15²/R = 1750
R = 15²/1750 = 0.129 Ω.
The resistance of the heater = 0.129 Ω.