Answer:
1.
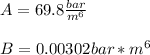
2.
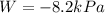
Step-by-step explanation:
Hello,
1. In this case, for the given p-V equation, one could use the two states to form a 2x2 linear system of equations in terms of A and B:
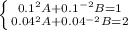
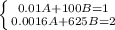
Whose solution by any method for solving 2x2 linear system of equations (elimination, reduction or substitution) is:
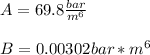
2. Now, for us to compute the work, we must first compute n, as the power relating the pressure and volume for this process:
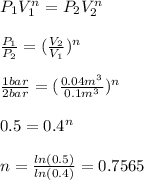
Now, we compute the work:
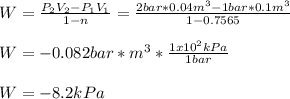
Regards.